What Mechanisms Are Associated With The Mucosa That Allow For Tissue Repair?
The immune system may exist viewed equally an organ that is distributed throughout the trunk to provide host defence against pathogens wherever these may enter or spread. Within the allowed system, a series of anatomically distinct compartments can be distinguished, each of which is peculiarly adapted to generate a response to pathogens present in a particular set of torso tissues. The previous part of the chapter illustrated the general principles underlying the initiation of an adaptive immune response in the compartment comprising the peripheral lymph nodes and spleen. This is the compartment that responds to antigens that accept entered the tissues or spread into the claret. A 2d compartment of the adaptive immune organisation of equal size to this, and located near the surfaces where almost pathogens invade, is the mucosal immune system (commonly described by the acronym MALT). Two further singled-out compartments are those of the body cavities (peritoneum and pleura) and the skin. Two central features ascertain these compartments. The first is that immune responses induced within ane compartment are largely confined in expression to that detail compartment. The 2d is that lymphocytes are restricted to detail compartments past expression of homing receptors that are bound past ligands, known as addressins, that are specifically expressed inside the tissues of the compartment. We will illustrate the concept of compartmentalization of the immune organization by considering the mucosal allowed arrangement. The mucosal surfaces of the body are particularly vulnerable to infection. They are thin and permeable barriers to the interior of the body because of their physiological activities in gas substitution (the lungs), food assimilation (the gut), sensory activities (optics, nose, mouth, and throat), and reproduction (uterus and vagina). The necessity for permeability of the surface lining these sites creates obvious vulnerability to infection and information technology is non surprising that the vast majority of infectious agents invade the human body through these routes.
A second important signal to deport in mind when because the immunobiology of mucosal surfaces is that the gut acts as a portal of entry to a vast array of foreign antigens in the grade of food. The immune organization has evolved mechanisms to avert a vigorous immune response to nutrient antigens on the i manus and, on the other, to notice and kill pathogenic organisms gaining entry through the gut. To complicate matters farther, most of the gut is heavily colonized by approximately tenxiv commensal microorganisms, which alive in symbiosis with their host. These bacteria are beneficial to their host in many ways. They provide protection against pathogenic bacteria by occupying the ecological niches for leaner in the gut. They also serve a nutritional role in their host by synthesizing vitamin K and some of the components of the vitamin B circuitous. However, in certain circumstances they can also cause disease, as we will see later.
10-thirteen. Mucosa-associated lymphoid tissue is located in anatomically divers microcompartments throughout the gut
The mucosa-associated lymphoid tissues lining the gut are known as gut-associated lymphoid tissue or GALT. The tonsils and adenoids grade a band, known as Waldeyer'south band, at the back of the mouth at the entrance of the gut and airways. They stand for big aggregates of mucosal lymphoid tissue, which oft become extremely enlarged in childhood considering of recurrent infections, and which in the past were victims of a faddy for surgical removal. A reduced IgA response to oral polio vaccination has been seen in individuals who take had their tonsils and adenoids removed, which illustrates the importance of this subcompartment of the mucosal immune system.
The other principal sites within the gut mucosal immune system for the induction of immune responses are the Peyer's patches of the modest intestine, the appendix (which is another frequent victim of the surgeon's knife), and solitary lymphoid follicles of the big intestine and rectum. Peyer's patches are an extremely important site for the induction of allowed responses in the pocket-size intestine and have a distinctive structure, forming domelike structures extending into the lumen of the intestine (see Fig. ane.10). The overlying layer of follicle-associated epithelium of the Peyer'south patches contains specialized epithelial cells. These have microfolds on their luminal surface, instead of the microvilli present on the absorptive epithelial cells of the intestine, and are known every bit microfold cells or 1000 cells. They are much less prominent than the absorptive gut epithelial cells, known as enterocytes, and form a membrane overlying the lymphoid tissue within the Peyer's patch. K cells lack a thick surface glycocalyx and do not secrete mucus. Hence they are adapted to interact directly with molecules and particles within the lumen of the gut.
Thou cells take upwards molecules and particles from the gut lumen past endocytosis or phagocytosis (Fig. 10.17). This fabric is then transported through the interior of the cell in vesicles to the basal cell membrane, where it is released into the extracellular space. This process is known as transcytosis. At their basal surface, the cell membrane of M cells is extensively folded around underlying lymphocytes and antigen-presenting cells, which have upward the transported fabric released from the One thousand cells and process it for antigen presentation.
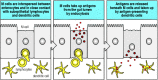
Figure ten.17
One thousand cells accept upwardly antigens from the lumen of the gut past endocytosis. The jail cell membrane at the base of these cells is folded around lymphocytes and dendritic cells inside the Peyer'due south patches. Antigens are transported through Yard cells by the process of transcytosis (more...)
Considering 1000 cells are much more accessible than enterocytes to particles inside the gut, a number of pathogens target M cells to proceeds access to the subepithelial space, even though such pathogens then find themselves in the heart of the adaptive allowed system of the intestine, the Peyer's patches. We will consider i of these pathogens in Section 10-19.
ten-14. The mucosal immune system contains a distinctive repertoire of lymphocytes
In addition to the organized lymphoid tissue in which induction of allowed responses occurs inside the mucosal immune organization, small foci of lymphocytes and plasma cells are scattered widely throughout the lamina propria of the gut wall. These represent the effector cells of the gut mucosal immune system. The life history of these cells is every bit follows. As naive lymphocytes, they sally from the primary lymphoid organs of bone marrow and thymus to enter the inductive lymphoid tissue of the mucosal immune system via the bloodstream. They may encounter foreign antigens presented within the organized lymphoid tissue of the mucosal immune system and become activated to effector status. From these sites, the activated lymphocytes traffic via the lymphatics draining the intestines, pass through mesenteric lymph nodes, and eventually wind up in the thoracic duct, from where they circulate in the claret throughout the entire body (Fig. ten.xviii). They reenter the mucosal tissues from the small claret vessels lining the gut wall and other sites of MALT, such as the respiratory or reproductive mucosa, and the lactating breast; these vessels limited the mucosal adressin MAdCAM-1. In this style, an allowed response that may be started by foreign antigens presented in a limited number of Peyer's patches is disseminated throughout the mucosa of the body. This pathway of lymphocyte trafficking is singled-out from and parallel to that of lymphocytes in the residuum of the peripheral lymphoid system (run across Fig. 1.11).

Effigy 10.18
Beefcake of mucosal allowed responses. The left panel shows the afferent immune response. Antigen from pathogenic micro-organisms is presented below mucosal surfaces to naive lymphocytes within organized mucosal lymphoid tissue, for example Peyer's patches. (more...)
The distinctiveness of the mucosal immune system from the rest of the peripheral lymphoid system is further underlined by the different lymphocyte repertoires in the different compartments. The T cells of the gut tin be divided into two types. 1 type bears the conventional α:β T-cell receptors in conjunction with either CD4 or CD8, and participates in conventional T-cell responses to strange antigens as discussed in earlier chapters. The second class is made up of T cells with unusual surface phenotypes such as TCRγ:δ and CD8α:α TCRα:β. The receptors of these T cells do not bind to the normal MHC:peptide ligands but instead bind to a number of dissimilar ligands, including MHC class IB molecules. These highly specialized T cells are abundant in the epithelium of the gut and have a restricted repertoire of T-cell receptor specificities. Different conventional T cells, many of these cells do not undergo positive and negative selection in the thymus (see Chapter 7), and limited receptors with sequences that accept undergone no or minimal divergence from their germline-encoded sequences. These cells may be classified in phylogenetic terms as being at the interface between innate and adaptive immunity.
T cells bearing a γ:δ receptor are peculiarly abundant in the gut mucosa compared with other lymphoid tissues. 1 subset of these γ:δ T cells in humans, which expresses a T-cell receptor that uses the Vδi gene segment, carries an activating C-blazon lectin NK receptor, NKG2D. This latter receptor binds to two MHC-similar molecules—MIC-A and MIC-B—that are expressed on abdominal epithelial cells in response to cellular injury and stress. The injured cells may then be recognized and killed by this subset of γ:δ T cells (Fig. ten.19). This illustrates one of the central roles of T cells, which is to patrol and survey the body, destroying cells that express an abnormal phenotype as a result of stress or infection.

Figure 10.19
T cells of the mucosal allowed system begetting γ:δ T-prison cell receptors and an activating NK receptor recognize and kill injured enterocytes. Infection or other injury to enterocytes, the epithelial cells lining the lumen of the gut, stimulates (more...)
The 5δane-containing receptor on these T cells may also play a part in allowing them to survey tissues for injured cells. Some human T cells expressing this receptor bind to CD1c, one of the isotypes of the CD1 family of MHC class I-similar molecules that we encountered in Department 10-v. This poly peptide, which shows increased expression on activated monocytes and dendritic cells, presents endogenous lipid and glycolipid antigens to some types of T cell. In response to antigen presentation past CD1c, these T cells secrete IFN-γ, which may have an important role in polarizing the response of conventional T cells bearing α:β receptors toward a THi response. This is closely analogous, although reverse in effect, to the polarization toward TH2 cells induced by secretion of IL-4 past NK 1.i+ T cells responding to CD1d discussed in Section 10-v.
A 2nd grouping of specialized mucosal T cells, so far only characterized in mice, express α:β T-prison cell receptors together with a CD8 α:α homodimer, instead of the normal CD8 α:β heterodimer that characterizes MHC class I-restricted cytotoxic T cells. These cells tin can exist constitute in the gut of mice lacking conventional MHC course I molecules, which shows that their development is not dependent on positive choice in the thymus by peptides bound to classical MHC course I molecules. They are, however, absent in mice defective expression of β2-microglobulin, which is necessary for the expression of MHC class IB molecules. The ligand recognized by these T cells in mice has been identified as the nonpolymorphic MHC class IB molecule known equally Qa-ii. These cells are likely to stand for a farther class of T cells that have a major role in maintaining the integrity of the gut mucosa by recognizing and destroying injured mucosal cells.
10-15. Secretory IgA is the antibiotic isotype associated with the mucosal immune system
The dominant antibody isotype of the mucosal allowed system is IgA. This class of antibody is found in humans in two isotypic forms, IgA1 and IgA2. The expression of IgA differs between the two main compartments in which it is establish—claret and mucosal secretions. In the claret, IgA is mainly found as a monomer and the ratio of IgA1 to IgA2 is approximately four:i. In mucosal secretions, IgA is almost exclusively produced as a dimer and the ratio of IgA1 to IgA2 is approximately 3:2. A number of common intestinal pathogens possess proteolytic enzymes that can assimilate IgA1, whereas IgA2 is much more resistant to digestion. The college proportion of plasma cells secreting IgA2 in the gut lamina propria may therefore be the effect of selective pressure by pathogens confronting individuals with depression IgA2 levels in the gut. The mechanism of isotype switching to IgA is discussed in Department nine-xiv.
At that place are special mechanisms for the secretion of polymeric IgA and IgM antibody into the gut lumen (encounter Section ix-xiii). Polymeric IgA and IgM are synthesized throughout the gut by plasma cells located in the lamina propria and are transported into the gut by immature epithelial cells located at the base of the abdominal crypts. These express the polymeric immunoglobulin receptor on their basolateral surfaces. This receptor binds polymeric IgA or IgM and transports the antibody past transcytosis to the luminal surface of the gut. Upon reaching the luminal surface of the enterocyte, the antibody is released into the secretions past proteolytic cleavage of the extracellular domain of the polymeric IgA receptor. Secreted IgA and IgM demark to the mucus layer overlying the gut epithelium where they tin bind to and neutralize gut pathogens and their toxic products (Fig. 10.xx).

Figure 10.20
The major antibody isotype present in the lumen of the gut is secretory polymeric IgA. This is synthesized by plasma cells in the lamina propria and transported into the lumen of the gut through epithelial cells at the base of the crypts. Polymeric IgA (more...)
10-16. About antigens presented to the mucosal allowed system induce tolerance
We are continuously exposed to a huge array of foreign antigens in the form of foods, merely these do non ordinarily induce an adaptive immune response. For example, IgA antibodies with high affinity to food antigens practise not ordinarily develop. This lack of response occurs despite the fact that the repertoire of lymphocyte antigen receptors has non been negatively selected to remove those specific for food antigens. This is considering, like any other strange antigen, food antigens do not play a role in the central mechanisms of lymphocyte tolerance to cocky, which are established in the thymus and bone marrow (meet Chapter 7).
The feeding of foreign antigens leads typically to a country of specific and active unresponsiveness, a miracle known as oral tolerance. Thus, no antibody response follows the feeding of a strange poly peptide such as ovalbumin, although a strong antibody response to this protein tin can be induced past injecting it subcutaneously, especially if an adjuvant is given every bit well. Yet, the feeding of ovalbumin is followed by a prolonged period during which the administration of ovalbumin by injection, fifty-fifty in the presence of adjuvant, elicits no antibiotic response. This suppression is antigen-specific, considering antibody responses to other injected antigens are not affected. These experiments show that there are antigen-specific mechanisms for suppressing peripheral immune responses to antigens delivered by oral cavity. The mechanisms of oral tolerance are partly understood but, before considering them, we will first hash out the contrasting immune responses that are seen in response to bacterial infections of the gut.
10-17. The mucosal allowed system tin can mount an immune response to the normal bacterial flora of the gut
We each harbor more than 400 species of commensal bacteria, which are present in the largest numbers in the colon and ileum. Despite the fact that these bacteria collectively weigh approximately 1 kg and outnumber us by approximately ten14 to 1, for virtually of the fourth dimension we cohabit with our abdominal bacterial flora in a happy symbiotic relationship.
One protective activity of our normal gut flora is that of contest against pathogenic leaner for space and nutrients, preventing their colonization of the gut (see Fig. ii.four). This activeness is dramatically illustrated by one of the adverse effects of antibiotics. Taking an antibiotic kills large numbers of commensal gut bacteria and thereby offers an ecological niche to bacteria that would not otherwise exist able to compete successfully with the normal flora and abound in the gut. One example of a bacterium that grows in the antibiotic-treated gut and can cause a astringent infection is Clostridium difficile; this produces ii toxins, which can crusade astringent bloody diarrhea associated with mucosal injury (Fig. 10.21).

Figure 10.21
Treatment with antibiotics causes massive death of the commensal bacteria that ordinarily colonize the colon. This allows pathogenic bacteria to proliferate and occupy an ecological niche that is unremarkably occupied by harmless commensal bacteria. Clostridium (more...)
At that place are some circumstances in which the normal bacterial inhabitants of the gut cause affliction, for example, following breakdown of the integrity of the mucosa lining the gut. This may occur post-obit poor blood menses in the gut, or following endotoxemia (see Chapter 2). In these circumstances, ordinarily innocuous gut leaner, such as nonpathogenic Escherichia coli, can cross the mucosa, invade the bloodstream, and cause fatal systemic infection. This illustrates the vital importance of the bulwark to infection provided past the mucosal surfaces of the body. The normal gut flora likewise becomes an important cause of systemic infection in patients with immunodeficiency. This illustrates the role of the adaptive immune system in host defense force against the flora of the gut, but also shows that this response does non result in the emptying of bacteria from the lumen of the gut, but rather a state resembling symbiosis.
The calibration of the normal immune response to gut bacteria is illustrated by the study of animals delivered by Caesarian section into a sterile surroundings in which there is no colonization of the gut by microorganisms. These are known every bit germ-gratuitous or gnotobiotic animals. These animals have marked reductions in the size of all secondary lymphoid organs and reduced levels of antibodies of all isotypes.
10-18. Enteric pathogens crusade a local inflammatory response and the development of protective immunity
In spite of the assortment of innate allowed mechanisms in the gut and strong competition from the ethnic flora, the gut is a frequent site of infection by pathogenic microorganisms. These include many species of viruses, enteric pathogenic bacteria including Salmonella, Yersinia, Shigella, and Listeria, and protozoa such as Entamoeba histolytica and Cryptosporidium. These organisms cause disease in many different ways, just there are certain common features of infection that are crucial to understanding how these pathogens stimulate an immune response by the host, in dissimilarity to the immunological tolerance shown to the foreign antigens ingested in food.
The most of import consequence of infection in the gut, as elsewhere in the torso, is the development of an inflammatory response. The release of cytokines and chemokines in this response is cardinal to the induction of an adaptive immune response. The inflammatory mediators stimulate the maturation of dendritic cells and other antigen-presenting cells, so that they express the co-stimulatory molecules that provide the additional signals for activation and expansion of naive lymphocytes.
Some intestinal pathogens infect enterocytes, the absorbent cells that line much of the intestine. Enterocytes do non act equally passive victims of infection only signal infection past releasing cytokines and chemokines. These include the chemokine IL-8, which is a potent neutrophil chemoattractant, and CC chemokines such as MCP-ane, MIP-1α and β, and RANTES, which are chemoattractants for monocytes, eosinophils, and T lymphocytes (see Fig. 2.33). In this way, the onset of infection triggers an influx of inflammatory cells and lymphocytes, leading to the induction of an immune response to the antigens of the infectious agent. Injury and stress to the enterocytes lining the gut may as well stimulate the expression of nonclassical MHC molecules, such as MIC-A and MIC-B (see Section 10-14). These human activity equally ligands to the receptors on γ:δ T cells at the base of the crypts, which impale the infected mucosal cell, thereby promoting repair and recovery of the injured mucosa.
A number of pathogens direct exploit the M cell as a means of invasion. Some viruses are transported through the Chiliad prison cell by transcytosis and from the subepithelial space are able to establish systemic infection. For example, from this site polio and retroviruses enter intestinal neuronal cells and spread to the central nervous system. HIV, which we talk over in detail in Chapter eleven, may apply a like route into the lymphoid tissue of the rectal mucosa, where it first encounters and infects macrophages.
Many of the most of import enteric leaner that cause infections in humans gain entry to the trunk through M cells. Invasion by this route delivers bacteria direct to the lymphoid system of the host. Depending on the pathogenicity of the organism and the forcefulness of the host adaptive immune response, infections that breach the gut mucosa may exist cleared with little tissue injury, cause a local inflammatory response, or invade the bloodstream or lymphatics and outcome in a systemic infection. Bacteria that specifically target M cells include Salmonella typhi, the causative agent of typhoid, and S. typhimurium, a major cause of bacterial food-poisoning. These bacteria crusade a brisk local and systemic inflammatory response associated with the induction of THi-type T-prison cell responses and antibody responses of the IgG and IgA classes.
10-19. Infection past Helicobacter pylori causes a chronic inflammatory response, which may cause peptic ulcers, carcinoma of the tum, and unusual lymphoid tumors
There is one exceptional bacterial infection of the stomach, in which the inflammatory and allowed response to the organism causes the disease instead of immigration the infection. Helicobacter pylori, which infects many millions of people around the world, adheres to the mucosa of the stomach and causes a local inflammatory response, with the release of IL-8 and the influx of leukocytes. In the majority of those infected there is no overt disease, only up to five% of infected people make either 1 of ii very different responses to the infection. In some, there is an excessive release of the hormone gastrin, which stimulates acrid production and the development of peptic ulcers. In others, chronic inflammation has the opposite effect, leading to atrophy of the breadbasket, which is associated with reduced acrid product and an increased risk of carcinoma of the stomach (Fig. 10.22). Rarely, lymphomas known as MALT lymphomas, because of their origin from the mucosa-associated lymphoid tissue, arise from the B lymphocytes that accumulate in the chronic inflammatory lymphoid infiltrates of the stomach. These are very extraordinary tumors, because some of them, despite being monoclonal proliferations with a transformed phenotype, are still dependent on antigenic or other inflammatory stimulation by H. pylori. These tumors may regress if the H. pylori infection is effectively eliminated by antibiotics alone.

Figure x.22
Helicobacter pylori infects the stomachs of many millions of people. In some, it stimulates the G cells of the breadbasket to secrete gastrin, which stimulates backlog acid production by the stomach, causing peptic ulceration. In others, it causes chronic (more than...)
10-xx. In the absence of inflammatory stimuli, the normal response of the mucosal immune organisation to foreign antigens is tolerance
We have seen that at that place are two possible and contrary outcomes of exposure to foreign antigens entering through the mucosa of the gut. These are tolerance in the example of food antigens, which is assorted with a vigorous antibiotic and T-cell response after exposure to pathogens. The essential difference between antigenic claiming past food compared with that by pathogens is that the pathogens cause inflammation, whereas nutrient does not. Both the antigens inside food and the antigens within pathogens are presented by antigenpresenting cells to T lymphocytes, but the contexts in which these two sources of antigen are presented are quite different.
Three different responses of T cells to the presentation of peptides derived from foods and other antigens delivered via the mucosa may business relationship for the phenomenon of oral tolerance. The first is the deletion of antigen-specific T cells by the induction of apoptosis, which has been establish to occur in experimental animals in response to oral intake of very big, and probably nonphysiological, doses of antigen. This is probably not the most important mechanism of oral tolerance, although it may contribute.
The second response is anergy, in which T cells presented with peptide in the absence of co-stimulatory signals become refractory to further stimulation with antigen (encounter Section 8-11). The development of anergy in response to a nutrient antigen was demonstrated by feeding ovalbumin to mice that had large numbers of T cells carrying a transgenically expressed T-cell receptor for an ovalbumin peptide epitope (run across Appendix I, Section A-46). Post-obit feeding of ovalbumin, T cells bearing the transgenic T-cell receptor could however be detected, but these were totally refractory to further stimulation by ovalbumin in vitro and in vivo, even when ovalbumin was injected systemically together with an adjuvant (Fig. 10.23).
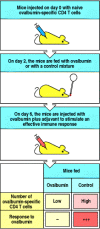
Figure 10.23
T-cell anergy following feeding of antigen. Mice were injected with CD4 T cells bearing a transgenic receptor specific for an ovalbumin peptide. Two days subsequently they were fed ovalbumin or a control protein. Four days later mice were injected with the relevant (more...)
The tertiary response involves the development of regulatory T cells, which tin can actively suppress antigen-specific responses following rechallenge with antigen. One subset of T cells has been described that produces IL-4, IL-10, and TGF-β on stimulation with antigen. These cells have been called TH3 cells. A similar population secretes TGF-β in an IL-10-dependent fashion and has been named TR1 (T regulatory ane cells). This pattern of cytokine secretion in response to antigen-specific stimulation inhibits the evolution of TH1 responses and is associated with low levels of antibody and about absent-minded inflammatory T-cell responses. The γ:δ T cells that are abundant throughout the mucosal immune system may too take a role in oral tolerance, because tolerance appears to exist reduced in mice lacking this subset of T lymphocytes.
Antigen-specific suppression is a form of oral tolerance that can be transferred experimentally to recipient animals by lymphocytes derived from animals that have been fed antigen. When an animal that has been injected with such lymphocytes is exposed to the same antigen for the outset time, these regulatory cells reply to the antigen and inhibit the responses of naive T cells in the recipient beast. This contrasts with anergic T cells, which cannot transfer oral tolerance following transfer to a naive animate being.
In order to understand the phenomenon of oral tolerance, information technology is essential to sympathise how orally delivered antigens are presented to T cells. Two routes of antigen presentation of soluble nutrient antigens accept been characterized that may induce T-cell responses favoring tolerance rather than allowed activation. The first is presentation of soluble food antigens past the antigen-presenting cells of the gut and other peripheral lymphoid organs. In the absence of inflammatory stimuli, antigen presentation by dendritic cells favors the induction of tolerance rather than T-cell activation. Dendritic cells in Peyer's patches have been shown to limited IL-10 and IL-4, in dissimilarity to similar cells in peripheral lymph nodes which express IFN-γ and IL-12. Withal, this heterogeneity of cytokine responses does non fully explain tolerance to food antigens. These may be detected in the bloodstream later on feeding and there is bear witness that the consecration of tolerance to food antigens takes place in lymph nodes and spleen as well as in the mucosal lymphoid organization. The second possible route of presentation of food antigens is by the enterocytes of the gut, which express MHC class I and MHC grade II molecules in the absenteeism of co-stimulatory molecules and thus may induce anergy on presenting antigens to intraepithelial lymphocytes.
We volition discuss each of these mechanisms of tolerance further in Chapter thirteen, where we consider how the loss of tolerance to self tissues may contribute to the evolution of autoimmune affliction. As we will see in Chapter 14, i of the strategies for treating allergy and autoimmune affliction is to attempt to dispense the nature of the antigen-specific response to stimulate T cells with the backdrop of such regulatory T cells.
Summary
The immune system can be divided into a series of functional anatomical compartments, of which the 2 well-nigh important are the peripheral lymphoid system made up of the conventionally studied spleen and lymph nodes, and the mucosal lymphoid system. Specific homing mechanisms for lymphocytes to each of these compartments serve to maintain a separate population of lymphocytes in each. The mucosal surfaces of the body are highly vulnerable to infection and possess a circuitous assortment of innate and adaptive mechanisms of amnesty. The adaptive immune system of the mucosa-associated lymphoid tissues differs from that of the residue of the peripheral lymphoid organisation in several respects. The types and distribution of T cells differ, with significantly greater numbers of γ:δ T cells in the gut mucosa compared with peripheral lymph nodes and blood. The major antibody blazon secreted beyond the epithelial cells lining mucosal surfaces is secretory polymeric IgA. The mucosal lymphoid system is exposed to a vast array of foreign antigens from foods, from the commensal bacteria of the gut, and from pathogenic microorganisms and parasites. No immune response can commonly be detected to food antigens. Indeed, soluble antigens taken past mouth may induce antigen-specific tolerance or antigen-specific suppression. In contrast, pathogenic microorganisms induce strong protective THone responses. Information technology is an important challenge to empathise these contrasting specific allowed responses. The key distinction between tolerance and the evolution of powerful protective adaptive immune responses is the context in which peptide antigen is presented to T lymphocytes in the mucosal immune system. In the absence of inflammation, presentation of peptide to T cells past MHC molecules on antigen-presenting cells occurs in the absenteeism of co-stimulation. By contrast, pathogenic microorganisms induce inflammatory responses in the tissues, which stimulate the maturation and expression of co-stimulatory molecules on antigen- presenting cells. This course of antigen presentation to T cells favors development of a protective TH1 response.
What Mechanisms Are Associated With The Mucosa That Allow For Tissue Repair?,
Source: https://www.ncbi.nlm.nih.gov/books/NBK27169/
Posted by: rockwellfook1949.blogspot.com


0 Response to "What Mechanisms Are Associated With The Mucosa That Allow For Tissue Repair?"
Post a Comment